


| MOQ: | 1kg |
| Price: | US $500/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 30 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 2000 Tons/Year |
Trichlorosilane (SiHCl3) is a chemical compound composed of one silicon atom bonded to three chlorine atoms and one hydrogen atom. It is a colorless, volatile liquid at room temperature. Here are some key points about trichlorosilane:
Chemical Composition: Trichlorosilane consists of one silicon (Si) atom bonded to three chlorine (Cl) atoms and one hydrogen (H) atom. Its chemical formula is SiHCl3.
Properties: Trichlorosilane is a volatile liquid that boils at around 31.8 degrees Celsius (89.2 degrees Fahrenheit) and has a melting point of -122 degrees Celsius (-187.6 degrees Fahrenheit). It is highly reactive and flammable. Trichlorosilane readily decomposes in the presence of moisture or water, releasing hydrogen gas and forming silicic acid.
Production: Trichlorosilane is primarily produced through the reaction of metallurgical-grade silicon (usually in the form of chunks or powder) with hydrogen chloride (HCl) gas at high temperatures:
Si + 3HCl → SiHCl3 + H2
This reaction typically occurs in the presence of a catalyst and is conducted in a closed system.
Uses: Trichlorosilane has various industrial applications:
Silicon Production: It is a key intermediate in the production of high-purity silicon. Trichlorosilane is used as a precursor in the Siemens process or the modified Siemens process, which involves the production of polycrystalline silicon for use in semiconductors and solar cells.
Chemical Synthesis: Trichlorosilane is used as a source of silicon in the synthesis of various silicon compounds, including silicones, silanes, and silicon carbide.
Hydrogen Production: Trichlorosilane can be used as a source of hydrogen gas through its reaction with water vapor or steam:
SiHCl3 + 3H2O → SiO2 + 3H2 + 3HCl
This reaction generates hydrogen gas for various industrial applications.
Metal Surface Treatment: Trichlorosilane is used as a surface treatment agent for metals to improve adhesion or provide a protective coating.
Safety Considerations: Trichlorosilane is a flammable liquid and should be handled with caution. It reacts vigorously with water, releasing hydrogen gas and forming corrosive silicic acid. Trichlorosilane vapors are also toxic and can cause respiratory irritation. Proper safety precautions, such as appropriate ventilation, protective equipment, and safe handling procedures, should be followed when working with trichlorosilane.
It's important to handle trichlorosilane safely and follow proper protocols to minimize risks associated with its reactivity, flammability, and potential health hazards.
Basic Info.
| Model No: | SiHCl3 | Quality | Electron Grade |
| Transport Package | Y-Cylinder, T-Drum, Tt, Tanker | Specification | 20L, 40L, 280L and customizable |
| Trademark | CMC | Origin | Suzhou, China |
| HS Code | 2812190091 | Production Capacity | 500ton/Month |
Specification:
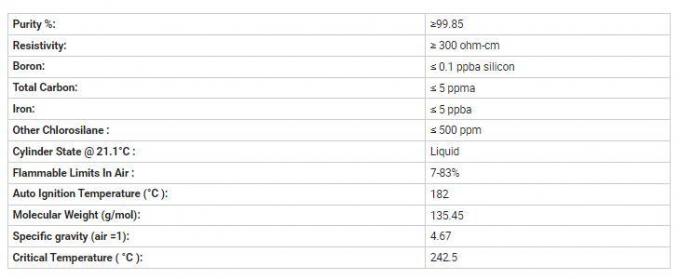
Detailed Photo
![]()



| MOQ: | 1kg |
| Price: | US $500/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 30 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 2000 Tons/Year |
Trichlorosilane (SiHCl3) is a chemical compound composed of one silicon atom bonded to three chlorine atoms and one hydrogen atom. It is a colorless, volatile liquid at room temperature. Here are some key points about trichlorosilane:
Chemical Composition: Trichlorosilane consists of one silicon (Si) atom bonded to three chlorine (Cl) atoms and one hydrogen (H) atom. Its chemical formula is SiHCl3.
Properties: Trichlorosilane is a volatile liquid that boils at around 31.8 degrees Celsius (89.2 degrees Fahrenheit) and has a melting point of -122 degrees Celsius (-187.6 degrees Fahrenheit). It is highly reactive and flammable. Trichlorosilane readily decomposes in the presence of moisture or water, releasing hydrogen gas and forming silicic acid.
Production: Trichlorosilane is primarily produced through the reaction of metallurgical-grade silicon (usually in the form of chunks or powder) with hydrogen chloride (HCl) gas at high temperatures:
Si + 3HCl → SiHCl3 + H2
This reaction typically occurs in the presence of a catalyst and is conducted in a closed system.
Uses: Trichlorosilane has various industrial applications:
Silicon Production: It is a key intermediate in the production of high-purity silicon. Trichlorosilane is used as a precursor in the Siemens process or the modified Siemens process, which involves the production of polycrystalline silicon for use in semiconductors and solar cells.
Chemical Synthesis: Trichlorosilane is used as a source of silicon in the synthesis of various silicon compounds, including silicones, silanes, and silicon carbide.
Hydrogen Production: Trichlorosilane can be used as a source of hydrogen gas through its reaction with water vapor or steam:
SiHCl3 + 3H2O → SiO2 + 3H2 + 3HCl
This reaction generates hydrogen gas for various industrial applications.
Metal Surface Treatment: Trichlorosilane is used as a surface treatment agent for metals to improve adhesion or provide a protective coating.
Safety Considerations: Trichlorosilane is a flammable liquid and should be handled with caution. It reacts vigorously with water, releasing hydrogen gas and forming corrosive silicic acid. Trichlorosilane vapors are also toxic and can cause respiratory irritation. Proper safety precautions, such as appropriate ventilation, protective equipment, and safe handling procedures, should be followed when working with trichlorosilane.
It's important to handle trichlorosilane safely and follow proper protocols to minimize risks associated with its reactivity, flammability, and potential health hazards.
Basic Info.
| Model No: | SiHCl3 | Quality | Electron Grade |
| Transport Package | Y-Cylinder, T-Drum, Tt, Tanker | Specification | 20L, 40L, 280L and customizable |
| Trademark | CMC | Origin | Suzhou, China |
| HS Code | 2812190091 | Production Capacity | 500ton/Month |
Specification:

Detailed Photo
![]()


